Complete Guide to RNA Sequencing
Understanding RNA-Seq Applications
Why perform RNA Sequencing (RNA-seq)? This technology provides a more precise view of transcriptomic profiling. It is not necessary to have a reference genome. Additionally, RNA-Seq can detect the presence of novel isoforms, alternatively spliced transcripts; potential gene fusion events can also be detected through analysis of RNA-seq data.
Sequencing the transcriptome isn’t simply running a sample through a series of instruments but capturing a snapshot of the true biological nature before RNA degrades. The technology excels at discovering new biological features and quantifying gene expression across your entire transcriptome with high sensitivity and dynamic range.
At Admera, we’ve found the difference between a breakthrough and a failed library preparation. We’ve put this guide together to bridge the gap between raw samples to meaningful data that actually determine your project’s success.
Complete Guide to RNA Sequencing: From Sample to Insights
RNA-Seq has revolutionized our ability to study gene expression and transcriptomics. Unlike traditional methods, RNA-Seq doesn't require a reference genome and can detect novel isoforms, alternatively spliced transcripts, and potential gene fusion events. Whether you're planning your first RNA-Seq experiment or optimizing your current workflow, this comprehensive guide covers everything you need to know.
Sample Preparation
RNA Extraction is the Critical Foundation
If you ask any bench scientist, RNA is looking for any reason to degrade. The fundamental instability of RNA means selecting the correct extraction kit is a strategic decision. High‑quality RNA isolation is widely regarded as one of the most critical steps in the entire RNA-Seq workflow. The quality of your extracted RNA directly determines the success of every downstream application—from library preparation to sequencing to final data analysis. Poor extraction can doom even the most carefully planned experiment, while high-quality extraction can salvage challenging sample types.
Why Extraction Quality Matters
Unlike DNA, RNA is inherently unstable due to ubiquitous RNases in the environment, on surfaces, and even on your skin. A single moment of RNase contamination can degrade your entire sample irreversibly. This fundamental vulnerability makes extraction technique and kit selection critical decisions that will impact your entire project.
Degraded RNA doesn't perform well in downstream applications. Even with modern protocols that can handle partially degraded material, starting with the highest quality RNA possible ensures optimal library complexity, better coverage across transcripts, and more reliable quantification of gene expression levels.
Choosing the Right Extraction Kit
The market offers numerous high-quality RNA extraction kits, each with specific strengths for different sample types and applications.
Depending on the sample type and storage conditions - our team uses our experience to select a kit for RNA extraction. Some kits may have a baseline recommendation or binding capacity/maximum input that may be used for an extraction attempt.
Since all extraction kits have a threshold for binding affinity and recommended inputs, combining samples may result in sample overload which will then affect nucleic acid quantity.
While silica-based columns are the industry standard, we’ve been moving toward 'inverse logic' systems like BioEcho’s EchoLUTION™.
BioEcho Life Sciences has emerged as an innovative solution with their EchoLUTION RNA purification kits separating nucleic acids from contaminants in a single step in under 15 minutes - without forcing binding to a solid surface. After lysis and a brief debris clearance, the lysate is loaded onto the EchoLUTION matrix, where impurities are retained and nucleic acids pass through directly. This is the inverse of silica-based logic.
EchoLUTION kits also eliminate the need for toxic chemicals like phenol and chloroform, making them safer for you. The room-temperature stable reagents simplify shipping and storage logistics. BioEcho's technology provides gentle purification that maintains RNA integrity while eluting valuable RNA for precious samples.
Extraction Considerations by Sample Type
Plant tissues require specialized approaches due to tough cell walls and high levels of polysaccharides and secondary metabolites that can interfere with purification. Select kits specifically designed for plant material or add additional cleanup steps.
Cell extractions depend heavily on cell number and origin. For approximately 1,000 cells, use low-input kits designed for minimal starting material. For around 500,000 cells, standard input kits work well. Remember that fibroblast and muscle cells yield less RNA than spleen, brain, or pancreatic cells.
FFPE samples present unique challenges due to crosslinking and degradation. Choose kits specifically validated for FFPE material, and expect lower yields and quality compared to fresh or frozen samples.
The DNase Treatment Decision
Many applications require DNase treatment to remove genomic DNA contamination despite this step causing up to 30-40% RNA loss. For poly-A selection library prep for coding RNA studies, DNA contamination is less concerning since the selection process removes it. However, ribosomal RNA (rRNA) depletion protocols, or non-coding & coding RNA studies, often benefit from DNase treatment. Weigh the tradeoff between DNA contamination risks and RNA loss based on your specific application and starting material.
Accepted Sample Types at Admera
The foundation of successful RNA-Seq begins with proper sample submission.
You can submit several types of samples:
Purified RNA remains the most straightforward option. Ship it on dry ice in RNase-free water. For room temperature shipping, use Omega BioTek RNA Transport or GenTegra RNA preservation systems. Always clarify whether your RNA has been DNase treated, as this affects downstream processing.
Fresh tissues should be snap frozen on dry ice or preserved in RNAlater for optimal results. The preservation method you choose impacts RNA quality, so plan your collection strategy carefully.
Cell samples require different handling depending on your library preparation approach. For extraction-based methods, send cells in Trizol. Low cell numbers (200-500 cells) can use SMART kits with 10X lysis buffer, bypassing extraction entirely. Read this guide for cell sorting. For moderate cell numbers (1,000-50,000+ cells), preservation buffers work well, with Qiagen Cell Protector recommended for long-term storage. Sorted cells should be sent in RLT+ buffer with beta-mercaptoethanol at a 100:1 ratio, using 5-7 times the sorting volume with a minimum of 350μL total.
FFPE samples present unique challenges due to degradation, requiring rRNA depletion protocols. Submit 5-10 slides at 10μM thickness for these specimens.
Expected RNA Yields
RNA extraction yields vary significantly by tissue type. High-yield tissues like liver, spleen, brain, lung, and heart can produce up to 20μg RNA per mg of tissue*. Moderate-yield tissues including embryo, kidney, lung, ovary, and thymus yield 0.05-2μg per mg. Low-yield tissues such as bladder, bone, and adipose produce less than 0.05μg per mg. Plant extractions can be particularly challenging due to tough cell walls requiring specialized protocols.
*Yield heavily dependent upon the amount and quality of starting materials. Storage conditions before arriving at Admera Health are critical to good output.Quality Control to Ensure Sample Integrity
Quantification Methods
Qubit (Fluorescence-based methods) provide the most accurate RNA concentration values. This is the primary form of quantification method in the next-generation sequencing market.
Nanodrop provides the traditional approach for RNA quantification. Pure RNA shows an A260/A280 ratio of 2.1, though values between 1.8-2.0 are acceptable. The A260/A230 ratio should exceed 1.8 to indicate minimal contamination from guanidine salts or phenol. However, Nanodrop is very sensitive and may pick-up off target signals that may skew quantification. As a result, our team would not recommend Nanodrop for next-generation sequencing applications as entirely reliable for RNA quantification.
Quality/Integrity Assessment
Bioanalyzers and TapeStations provide quantitative integrity assessment through the RNA Integrity Number (RIN). The DV200 metric, measuring the percentage of fragments above 200bp, becomes critical for degraded samples—values at or below 30% significantly reduce library construction success.
Using degraded RNA in downstream applications runs a risk of unsuccessful downstream applications, making quality assessment essential before investing in library preparation. For samples with RIN values between 5-7, expect possible 5' bias, though gene expression results typically remain unaffected. DNase treatment, when necessary, causes 30-40% RNA loss.
Admera has a specific expertise in working with challenging samples through our optimized library preparation protocols.
Library Preparation Strategies
Poly-A Selection
Poly-A enrichment serves as the first choice for most applications, generating the highest percentage of reads mapping to protein-encoding genes. This method requires high-quality RNA with RIN ≥7 and minimal degradation. Since total RNA samples contain up to 90% ribosomal sequences while mRNAs comprise only 1-2%, enrichment proves highly desirable.
For long-read sequencing applications, we recommend starting with high-quality RNA with RIN ≥ 7 for optimal full-length transcript recovery. The goal is to characterize coding transcriptome, the cDNA synthesis process must be optimized for full-length production. Any fragmentation or incomplete synthesis at this stage may lead to truncated reads which may misrepresent true transcript length.
Ribosomal RNA (rRNA) Depletion
When transcripts lack polyA tails (bacterial RNA) or you need to retain long non-coding RNAs (lncRNA), rRNA depletion becomes necessary. This approach also enables analysis of slightly degraded samples with RIN scores of 5 or higher.
There are many options to accommodate low input, low quality samples like what you may see with FFPE samples. DNase treatment is recommended for rRNA depletion samples, particularly exosome samples that tend toward high degradation.
Small RNA and Specialized Applications
Small RNA sequencing requires careful distinction between small RNA (20-200bp) and microRNA (<20bp). Library preparation kits in the market require high-quality RNA (RIN>7) with input ranging from 2ng to 1μg, with maximum volumes for library preparation.
Circular RNA (circRNA) sequencing requires a minimum of 2μg input and needs rRNA depletion. RNase R treatment before library preparation remains optional, depending on experimental goals.
Library Characteristics
We often get asked why we can't just sequence everything in the tube.
The reality is that chemical fragmentation sets a 'hard floor' and 'hard ceiling' on what we can capture. If your target is below 100nt, standard workflows will miss it, while anything over 400nt risks adapter dimer contamination. When we configure a run for a target insert of 200bp, we are balancing the physical limitations of the sequencers with the biological complexity of your sample.
It’s a trade-off and knowing where to draw those lines is part of the Admera expertise.
Typical RNA-Seq workflows cannot capture fragments below 100nt or above 400nt due to chemical fragmentation limitations and adapter dimer contamination risks. Target insert size runs approximately 200bp, with final library size around 400-430bp and inserts of 280-310bp.
Stranded Library Considerations
Strand information is maintained through adapter directionality. First-strand directional workflows like with NEBNext or TruSeq produce Read 2 from the original RNA strand and Read 1 from the opposite strand, degrading the original strand through dUTP incorporation. Second-strand directional protocols such as that with SMARTer Stranded generate Read 1 from the original RNA strand and Read 2 from the opposite strand, preserving directionality through differential adapter ligation.
Sequencing Recommendations
Illumina Short-Read Sequencing
Illumina 2x150 NovaSeq platforms provides the standard approach for most RNA-Seq applications. Depth requirements vary by application: PolyA selection needs 40M paired-end reads (20M per direction), rRNA depletion requires 80M paired-end reads (40M per direction), and small RNA sequencing needs 10M paired-end reads (5M per direction). TCR sequencing requires specialized 600-cycle configurations.
PacBio Iso-Seq Long-Read Sequencing
For full-length transcript sequencing, PacBio Revio systems provide the current state-of-the-art. Iso-Seq library preparation begins with high-quality, full-length cDNA Kinnex array formation followed by SMRTbell adapter ligation. The technology uses circular consensus sequencing (CCS) to achieve high accuracy (>99.9%) despite the longer read lengths.
Sequencing Depth for Iso-Seq
The required depth depends on your research goals. For comprehensive isoform discovery in complex transcriptomes, aim for 4-8M segmented reads per sample. For targeted analysis or simpler transcriptomes aim for 0.8M to 4M segmented reads per sample. Unlike short-read sequencing where depth correlates with quantification accuracy, Iso-Seq depth primarily affects the completeness of isoform discovery.
Iso-Seq Workflow Considerations:
Library prep includes cDNA size selection to optimize for your transcripts of interest (typically 1-2 kb bins and up to 10 kb bins)
Multiplexing options allow pooling multiple samples, though this reduces per-sample read depth
Data analysis requires specialized pipelines to process CCS reads, remove artifacts, and collapse redundant isoforms
Integration with short-read data can improve quantification accuracy while maintaining isoform resolution
Experimental Design Considerations
Sample Size and Controls
We recommend for differential expression studies to have at least triplicate samples, though duplicate samples may suffice for some applications. Consider incorporating controls like PhiX or ERCC spike-ins, adding spike-ins prior to shipping or as part of library preparation. In-line DNA controls found in TruSeq protocols help identify library preparation failure points.
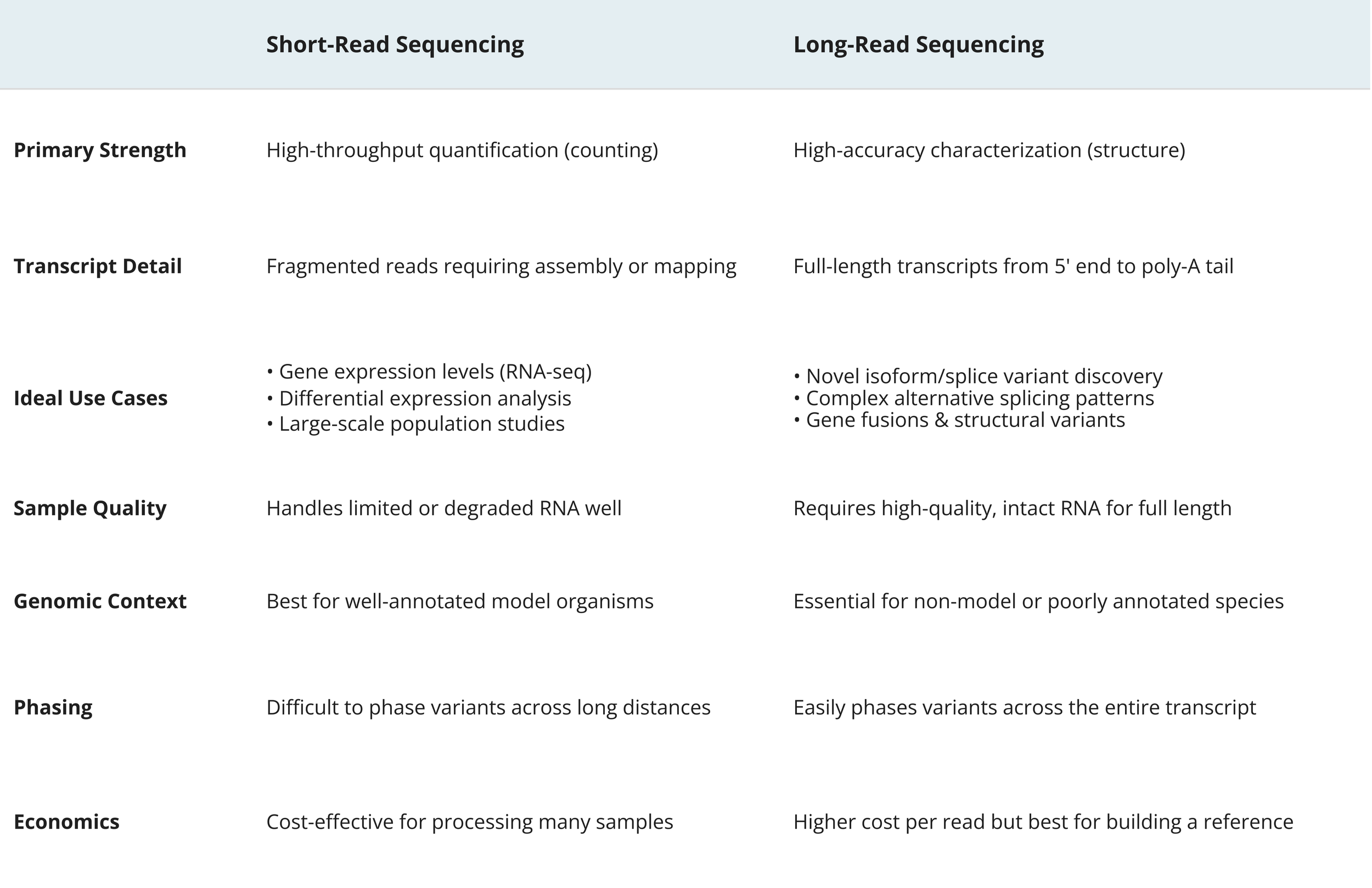
Short-Read vs. Long-Read Sequencing
Short-read sequencing using Illumina platforms has dominated the RNA-Seq landscape for over a decade, offering high accuracy, deep coverage, and cost-effectiveness. These platforms excel at quantifying gene expression and detecting differential expression between conditions. However, short reads (typically 150bp paired-end) must be computationally assembled to reconstruct full-length transcripts, which can introduce ambiguity when dealing with complex isoforms or highly similar gene families.
Long-read sequencing has revolutionized our ability to characterize transcriptomes at the isoform level. PacBio's Iso-Seq (Isoform Sequencing) technology sequences full-length transcripts in single reads, eliminating the need for computational assembly and providing unambiguous isoform identification.
PacBio Iso-Seq Full-Length Transcript Analysis offers several transformative advantages.
Complete Isoform Resolution - Rather than inferring transcript structure from short fragments, Iso-Seq reads span entire mRNA molecules from the 5' cap to the 3' poly(A) tail. This eliminates ambiguity in identifying alternatively spliced isoforms, even those differing by a single exon.
Novel Isoform Discovery - The full-length nature of Iso-Seq reads enables confident identification of previously unannotated isoforms, alternative transcription start sites, alternative polyadenylation sites, and complex splicing patterns that short reads might miss or misassemble.
Fusion Transcript Detection - Gene fusions are captured in single reads, providing definitive evidence of fusion events without the assembly artifacts that can complicate short-read fusion detection.
Allele-Specific Expression - Long reads can phase variants across entire transcripts, enabling precise measurement of allele-specific isoform expression.
Simplified Analysis - Without the need for transcript assembly, Iso-Seq data analysis becomes more straightforward and less computationally intensive for isoform-level questions.
When to Choose Each Platform
Sequencing Platform Comparison Table
Complementary Approaches - Many researchers now employ hybrid strategies, using Iso-Seq to establish a comprehensive isoform catalog for their organism or tissue of interest, then using Illumina sequencing for cost-effective quantification across experimental conditions. This combination leverages the strengths of both technologies.
Bioinformatic Deliverables
Standard RNA-Seq analysis provides QC statistics, sequence alignments, alignment quality summaries, normalized counts, gene feature counting, differential expression analysis, and GO/KEGG pathway analysis. MicroRNA projects additionally include plots of novel and known miRNA structures with read mapping signatures.
Check out demo reports here.
Conclusion
Successful RNA sequencing projects rely on the right expertise. Admera’s expertise is the difference between a routine RNA-seq project and an exceptional experience that leaves you with high-quality data for your next big discovery.
Understanding your biological question helps determine the appropriate library preparation method. That may mean utilizing the poly-A selection for mRNA studies, rRNA depletion for comprehensive transcriptome analysis including non-coding RNAs, or specialized protocols for small RNA or circular RNA investigations. By following these guidelines for sample quality, library preparation, and sequencing depth, you'll maximize your chances of generating high-quality, biologically meaningful data.
The field is moving fast, with new protocols emerging all the time. You don’t have to navigate those changes alone. Let’s connect early in your planning phase to make sure your experimental design isn't just "current," but truly optimized for the results you need.
Contact us to inquire about your RNA-seq project, our team is happy to help design and execute the right strategy for your project.